Articles from PharmaJet

PharmaJet®, a company that strives to improve the performance and outcomes of injectables with its needle-free injection technology, today announced the publication of a study demonstrating the benefits of the PharmaJet Tropis needle-free system compared with N/S for ID administration of fractional dose poliovirus vaccine (fIPV). The study entitled Tropis needle-free injector for fractional-dose IPV administration: A pilot study for integration into routine immunization services in Cuba is published in Vaccine.1
By PharmaJet · Via Business Wire · May 1, 2025

PharmaJet®, a company that strives to improve the performance and outcomes of injectables with its needle-free injection technology, will present insights from several DNA cancer vaccine partner trials using PharmaJet’s Precision Delivery Systems™ at the World Vaccine Congress 2025 on April 23, 2025 at 12:25 PM ET.
By PharmaJet · Via Business Wire · April 17, 2025

PharmaJet®, a company that strives to improve the performance and outcomes of injectables with its enabling needle-free injection technology, today announced that their Tropis intradermal (ID) delivery system was used in the latest World Health Organization’s (WHO) polio eradication campaign in Pakistan in February 2025. The supplemental immunization activity (SIA) campaign for children between 4 and 59 months of age was implemented in two regions in the Quetta block (Pishin, Quetta, Kila Abdullah, and Chaman) and Karachi (Central Karachi, East, East-Gujro, Keamari, Korangi, Malir, and West). Tropis was used to deliver fractional dose inactivated polio vaccine (fIPV) in parallel to oral polio vaccine (OPV) administration as part of a WHO recommended strategy to boost humoral immunity.
By PharmaJet · Via Business Wire · March 13, 2025

PharmaJet®, a company that strives to improve the performance and outcomes of injectables with its needle-free injection technology, today announced the npj Vaccines publication of Gennova Biopharmaceutical’s post hoc analysis of their Phase 3 clinical study, which evaluated the cellular immune breadth induced by their self-amplifying (samRNA) vaccine (GEMCOVAC-OM) administered as a booster with Tropis ID. The analysis, entitled Cellular immune breadth of an Omicron-specific, self-amplifying monovalent mRNA vaccine booster for COVID-19 showed that GEMCOVAC-OM, administered exclusively with Tropis, generated high levels of memory B-cell and T-cell responses, important elements of an effective immune reaction to new variants.
By PharmaJet · Via Business Wire · April 2, 2025

PharmaJet®, a company that strives to improve the performance and outcomes of injectables with its enabling technology, today announced that they will share the latest routine immunization study results at the 61st Annual Medical Conference and International Health Exhibition to be held on February 21-22, 2025 at the Haile Grand in Addis Ababa, Ethiopia. The presentation, entitled Intradermal needle-free vaccine delivery to reduce Ethiopia’s immunization costs and improve coverage, will be presented by Paul LaBarre, Vice President Global Business Development, PharmaJet. This event will bring together physicians from across the nation, along with representatives from Ethiopian Medical Association chapter offices, medical specialty societies, medical schools, the Ministries of Health (MoH) and Education, sister health professional associations, and partners.
By PharmaJet · Via Business Wire · February 19, 2025

In the headline and first paragraph, first sentence of release dated Dec. 10, 2024, the publication's name should read: NPJ Vaccines (instead of Nature).
By PharmaJet · Via Business Wire · December 11, 2024

PharmaJet®, a company that strives to improve the performance and outcomes of injectables with its enabling needle-free technology, today announced their upcoming poster presentation on November 13, 2024 at the International mRNA Health Conference 2024. The PharmaJet poster (#32), entitled Modulating Immune Responses to mRNA Vaccines Using Needle-Free Delivery Technologies, will be presented by Dr. Carmen Ledesma-Feliciano, DVM, PhD, DACLAM, Scientific Affairs Manager, PharmaJet. The conference brings together industry and academic professionals to explore the rapidly advancing science and business of mRNA medicines and features poster presentations highlighting the latest advancements in mRNA technology.
By PharmaJet · Via Business Wire · November 12, 2024
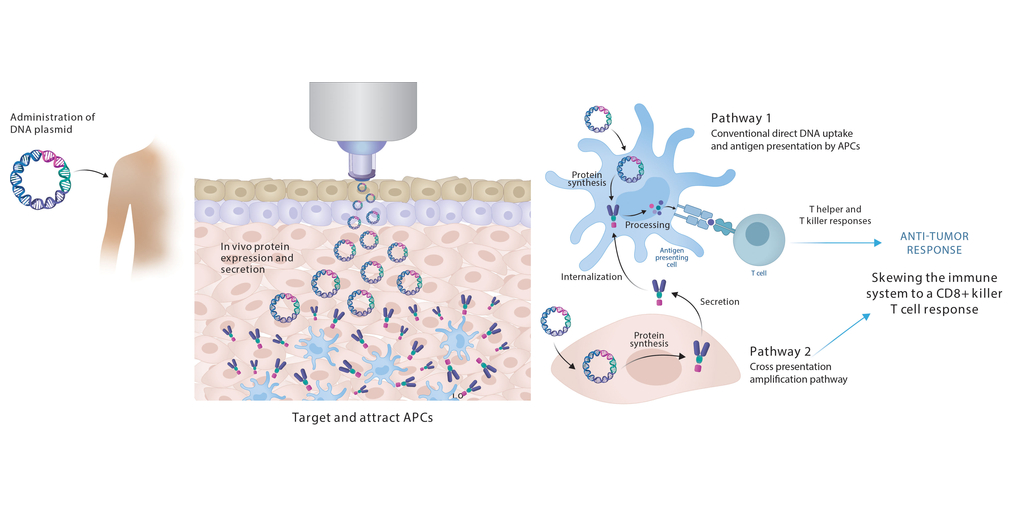
PharmaJet®, a company that strives to improve the performance and outcomes of injectables with its enabling needle-free technology, today announced their upcoming poster presentation on November 8, 2024 at the Society of Immunotherapy of Cancer (SITC) conference. The poster (#741), entitled Modulating Immune Responses to Therapeutic Cancer Vaccines through Precision Delivery Technologies, will be presented by Gregg Wilson, PhD, RN, Director of Medical and Scientific Affairs, PharmaJet. The SITC conference will be held at the George R. Brown Convention Center in Houston, Texas, bringing together leading cancer immunotherapy researchers, clinicians, scientists and industry leaders in the oncology field.
By PharmaJet · Via Business Wire · November 5, 2024
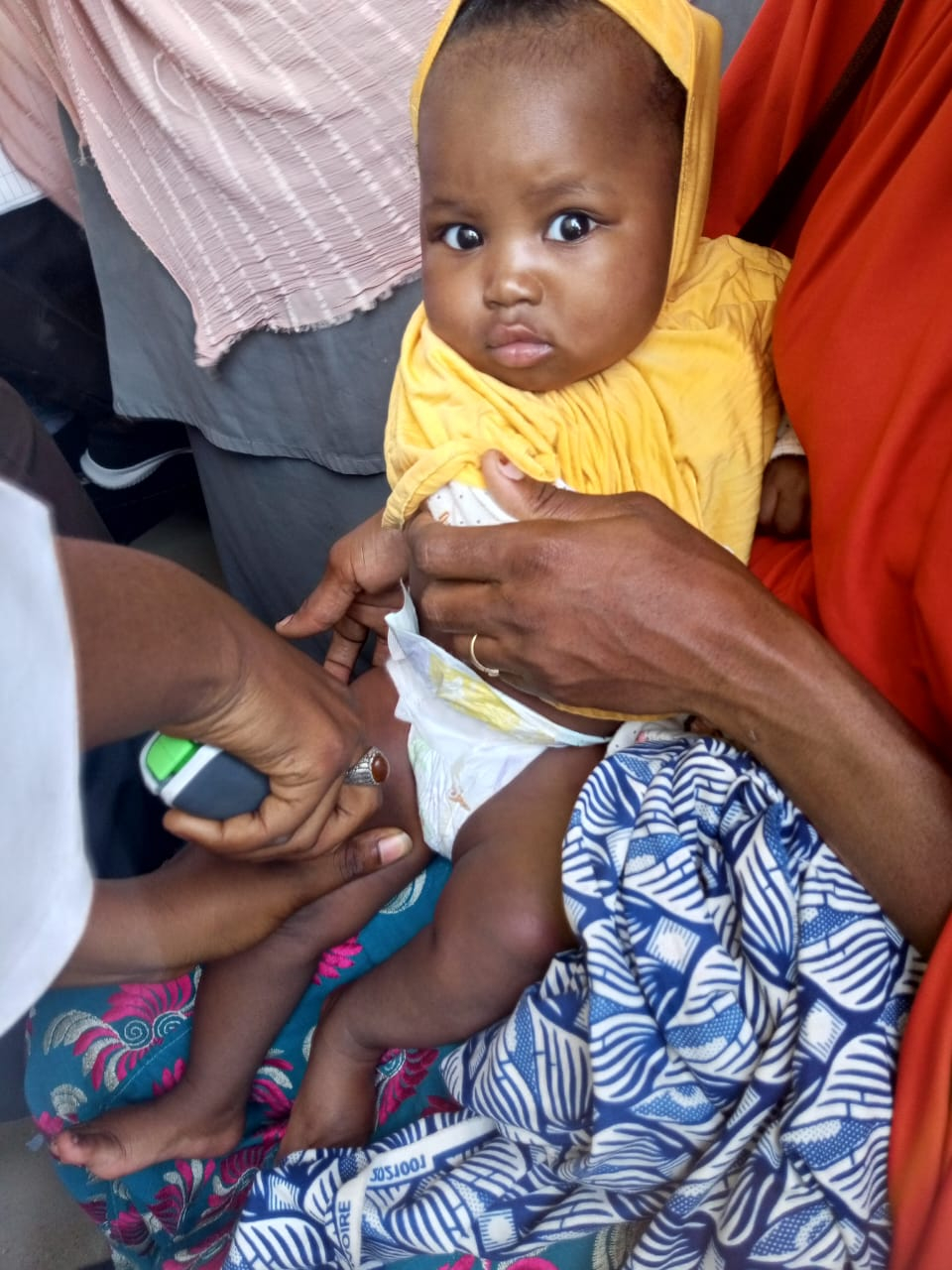
PharmaJet®, a company that strives to improve the performance and outcomes of injectables with its enabling needle-free technology, today announced the results of an implementation study in Nigeria that evaluated the impact on coverage, costs, and acceptability of intradermal fractional inactivated poliovirus vaccine (fIPV) administration using their Tropis® ID Needle-free System, compared to the standard of care (full-dose, IPV intramuscular delivery with needle and syringe). The study results were presented at an event attended by experts from the Nigeria Federal Ministry of Health (FMOH), WHO, UNICEF, and the United States Agency for International Development (USAID). The study principal investigators from the Nigeria National Primary Health Care Development Agency (NPHCDA), Jhpiego, PATH, and Sydani Group reported increased coverage, decreased program costs, and a strong preference for routine immunization using Tropis among healthcare workers1:
By PharmaJet · Via Business Wire · October 24, 2024

PharmaJet®, a company that strives to improve the performance and outcomes of injectables with its enabling technology, today announced that they will share their latest research results at the World Vaccine Congress – Europe on October 29, 2024 at 1:40 pm GMT. The presentation, entitled Improving Performance of Vaccines with Needle-free Technology will be presented by Nathalie Landry, Chief Scientific Officer, PharmaJet.
By PharmaJet · Via Business Wire · October 17, 2024

PharmaJet®, a company that strives to improve the performance and outcomes of injectables with its enabling technology, today announced that Wouter Latour, M.D., has joined the company as Chief Executive Officer (CEO). Latour also joins the PharmaJet board of directors. He succeeds Chris Cappello who has recently been elected Vice-Chairman of the Board and will remain active in the business, largely focused on strategic partners and supporting engineering/operations.
By PharmaJet · Via Business Wire · October 2, 2024

PharmaJet®, a company that strives to improve the performance and outcomes of injectables with its enabling technology, today announced that it has entered into a long-term license and supply agreement with Scancell Holdings plc (LSE – SCLP.L) to use the US FDA 510(k)-cleared / CE-marked PharmaJet Stratis® Intramuscular (IM) Needle-free System for the delivery of its advanced melanoma DNA vaccine (Immunobody® SCIB1/iSCIB1+).
By PharmaJet · Via Business Wire · September 17, 2024

PharmaJet®, a company that strives to improve the performance and outcomes of injectables with its innovative delivery systems, today announced that their Tropis® Intradermal (ID) Needle-free System will be used in a house-to-house polio immunization campaign. With an aim of significantly reducing the immunity gap against type-2 poliovirus, the campaign will be conducted in two rounds. In each round, children 4-59 months of age will receive the needle-free polio vaccine and novel oral polio vaccine with a goal of achieving 95% coverage. The campaign, a collaboration of the African Field Epidemiology Network (AFENET), WHO, UNICEF, BMGF, GAVI and CDC, is targeting over 170,000 children in 4 districts in Banadir, Somalia.
By PharmaJet · Via Business Wire · June 27, 2024
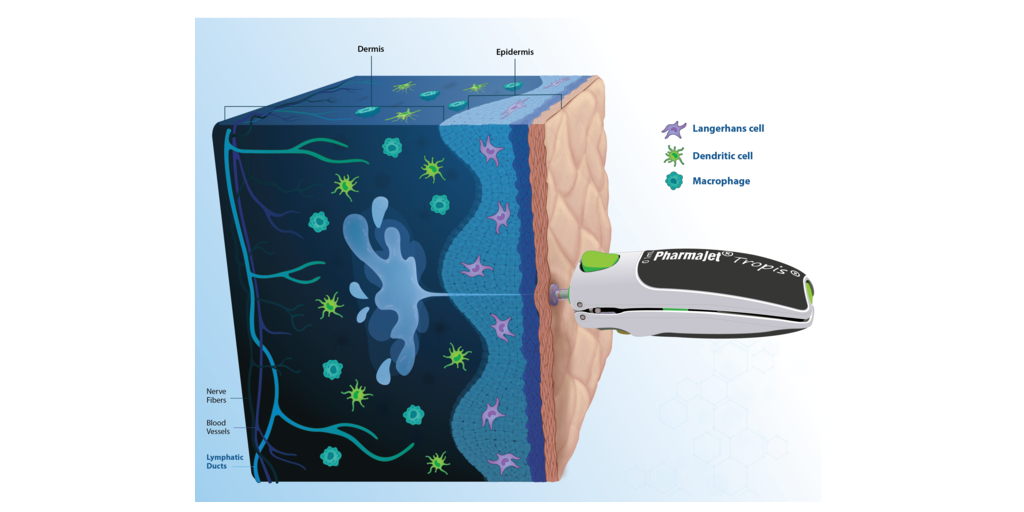
PharmaJet®, a company that strives to improve the performance and outcomes of medicines with its innovative delivery systems, today announced the Nature publication1 of Gennova Biopharmaceutical’s Phase 2/3 clinical trial conducted to evaluate the safety and immunogenicity of its novel samRNA-based Covid-19 vaccine booster. The results demonstrated that GEMCOVAC-OM, administered exclusively with Tropis, is well-tolerated with no related serious adverse events and significantly boosts immune responses against the Omicron variant. Furthermore, the publication cited that the self-amplifying, thermostable mRNA platform delivered intradermally with Tropis provides a framework for next-generation vaccines that can improve accessibility and global equity.
By PharmaJet · Via Business Wire · April 24, 2024
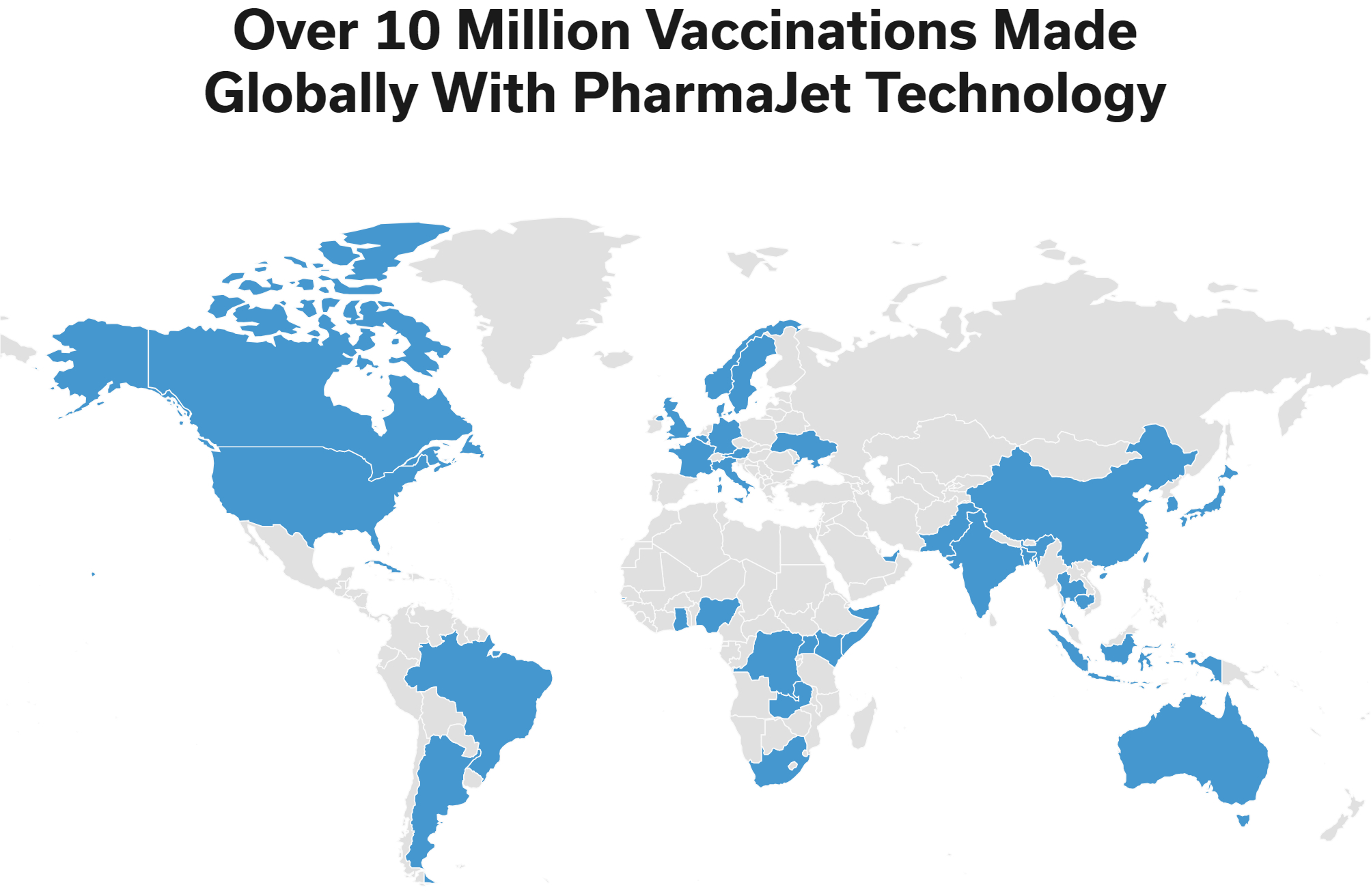
PharmaJet®, a company that strives to improve the performance and outcomes of medicines with its innovative delivery systems, today announced the formation of two Scientific Advisory Boards (SAB) comprised of leading pharmaceutical industry experts in immunology, cancer, research, and policy shaping. The Boards will provide strategic guidance and expertise to help PharmaJet advance its vision to better activate the immune system with its unique technology and accelerate its partnering strategy with vaccine and pharmaceutical companies.
By PharmaJet · Via Business Wire · April 1, 2024

PharmaJet®, a company that has developed and commercialized a needle-free platform to more effectively administer drugs and biologics, today announced the start of the first human clinical trial for a Venezuelan Equine Encephalitis (VEE) vaccine delivered with PharmaJet Precision Delivery Systems. The Phase 1 study, sponsored by PharmaJet, aims to identify the optimal dose, vaccination schedule and delivery system most suitable for use in subsequent broader clinical evaluations of the VEE DNA vaccine candidate.
By PharmaJet · Via Business Wire · November 16, 2023

PharmaJet®, a company that engineers precision delivery systems that overcome the challenges of vaccines and pharmaceuticals delivery, today announced that it received a strategic investment from the Daicel Corporation. This investment coincides with Daicel’s announcement of the establishment of a new company, Daicel Medical Ltd., and reinforces Daicel’s commitment to growth in this critical area of healthcare.
By PharmaJet · Via Business Wire · October 31, 2023
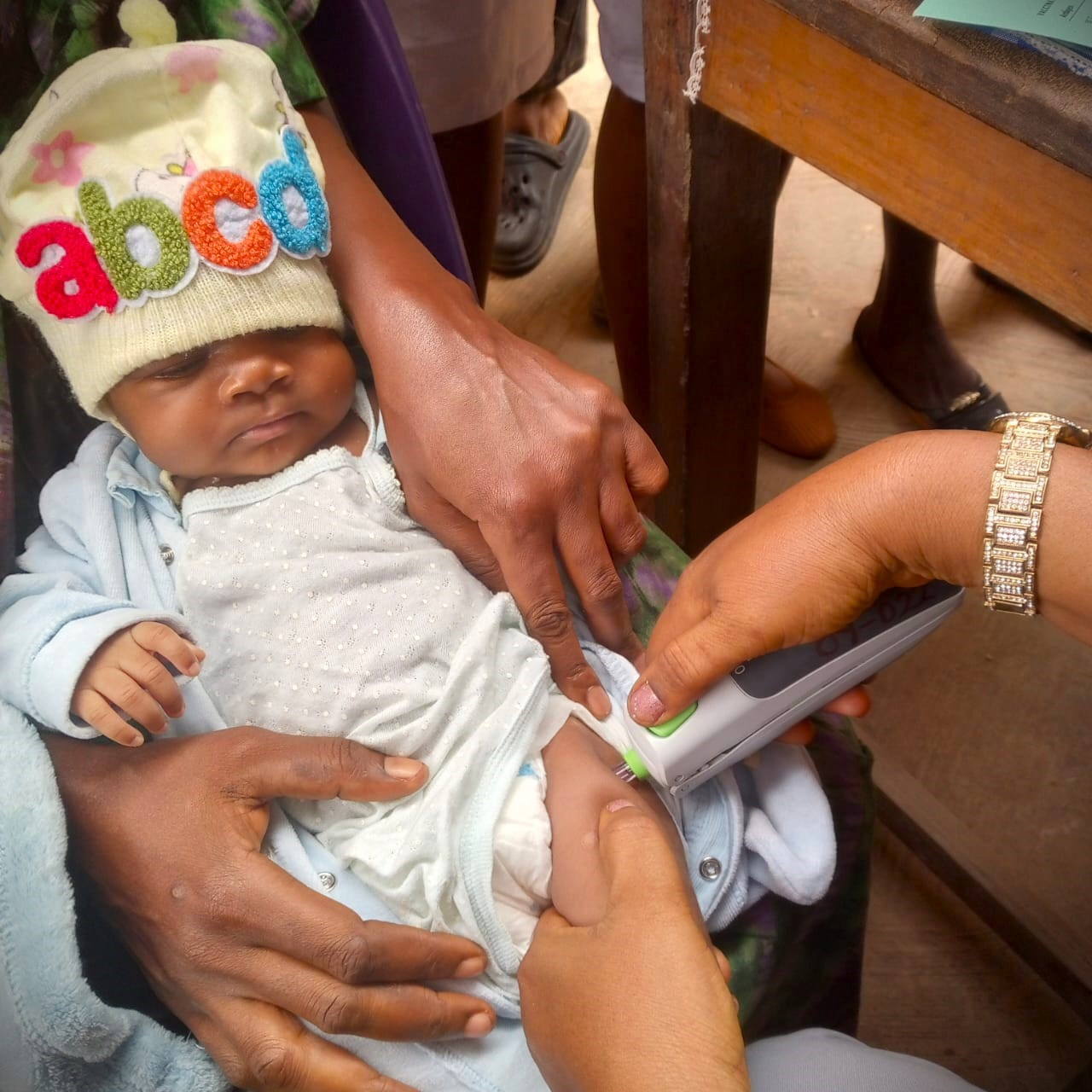
PharmaJet®, a company that engineers precision delivery systems that overcome the challenges of vaccines and pharmaceuticals delivery, today announced the implementation of a study in Nigeria to evaluate the impact of intradermal vaccine administration of fractional inactivated poliovirus vaccine (fIPV) using their Tropis® ID Needle-free Injection System (NFIS). The study, in collaboration with the National Primary Health Care Development Agency (NPHCDA), Jhpiego, PATH and the Sydani Group, is assessing coverage rates, and the potential for reduced costs, associated with using Tropis for fIPV delivery, as compared to the current standard of intramuscular delivery of full dose IPV using needle and syringe. Partners are also evaluating the acceptability and feasibility of using needle-free from the healthcare worker and caregiver perspective.
By PharmaJet · Via Business Wire · October 24, 2023

PharmaJet®, a company that engineers precision delivery systems that overcome the challenges of vaccines and pharmaceuticals delivery, today announced that its partner, Scancell, has received positive data from the first stage in its Phase 2 clinical study for treatment of patients with unresectable advanced melanoma. The vaccine is delivered by needle-free injection with the PharmaJet Stratis® System, which patients prefer over needle and syringe delivery.
By PharmaJet · Via Business Wire · September 28, 2023

PharmaJet,® a company engineering Precision Delivery Systems™ that overcome the challenges of vaccines and pharmaceuticals delivery, today announced that Nanette Cocero, Ph.D., has been appointed to its Board of Directors. Dr. Cocero is a recognized leader in the global pharmaceutical industry with broad commercial and business experience coupled with an extensive clinical and scientific background.
By PharmaJet · Via Business Wire · July 11, 2023

PharmaJet®, a company engineering precision delivery systems that overcome challenges of nucleic acid vaccine delivery, today announced that its Tropis® System is now the exclusive intradermal delivery system for two authorized novel Covid-19 vaccines:
By PharmaJet · Via Business Wire · June 28, 2023

PharmaJet,® a company engineering Precision Delivery Systems™ that overcome the challenges of vaccines and pharmaceuticals delivery, today announced that Marie Mazur, PharmD, has been appointed Chair of the Board of Directors. She replaces Ron Lowy who had chaired the Board since 2011.
By PharmaJet · Via Business Wire · June 6, 2023

PharmaJet®, a company engineering precision delivery systems that overcome the challenges of vaccine delivery, today announced their support for the World Health Organization’s (WHO) polio eradication campaigns in Pakistan starting in May 2023. PharmaJet is providing an additional 5 million syringes to the WHO for a supplemental immunization activity (SIA) campaign which will be conducted in two phases in the Southern districts of Khyber Pakhtunkhwa (KP) Province located in the north-west region of Pakistan, and in the Quetta Block located in Northern Balochistan Province near the Pakistan-Afghanistan border. The PharmaJet Tropis Precision Delivery System™ (PDS) was selected based on its proven cost savings, ease of training, and improved immunization coverage benefits.
By PharmaJet · Via Business Wire · May 23, 2023

PharmaJet,® a company engineering Precision Delivery Systems™ that may overcome the challenges of vaccines and pharmaceuticals delivery today announced that Nathalie Landry, has been appointed Chief Scientific Officer.
By PharmaJet · Via Business Wire · May 2, 2023

PharmaJet®, a company engineering Precision Delivery Systems™ (PDS) that overcome the challenges of vaccine delivery, today announced that it received a scope expansion and 3-year extension of its multi-year agreement with the Joint Science and Technology Office of the U.S. Defense Threat Reduction Agency (DTRA). The expansion award, worth over $4 million, will be used to further advance the clinical assessment of its Venezuelan Equine Encephalitis Virus (VEEV) DNA candidate vaccine in combination with either Tropis Intradermal (ID) or Stratis Intramuscular (IM) Systems.
By PharmaJet · Via Business Wire · April 18, 2023

Results of a comprehensive review of published scientific literature comparing data associated with various DNA vaccine delivery methods will be presented at the World Vaccine Congress 2023 on April 5, 2023 at 5:10 pm ET.
By PharmaJet · Via Business Wire · April 4, 2023

Following assessment of the primary endpoints of the Phase II/III study, PharmaJet partner Gennova Biopharmaceuticals Limited has submitted data for its mRNA-based Omicron specific Covid-19 booster shot for Emergency Use Authorization (EUA) to the office of the Drug Controller General of India (DCGI). The submission corresponds with an increase in COVID-19 cases in India1 and is the first booster in India targeted specifically for the Omicron variant. The vaccine, GEMCOVAC-OM, will be delivered exclusively with the PharmaJet Tropis Precision Delivery System (PDS).
By PharmaJet · Via Business Wire · April 3, 2023

PharmaJet®, a company engineering precision delivery systems that may overcome the challenges of nucleic acid vaccine delivery, today announced that its partner, Scancell, a developer of novel immunotherapies for the treatment of cancer and infectious diseases, reported positive results from their Phase 1 COVIDITY clinical trial. The trial was conducted at the University of Cape Town Lung Institute in South Africa to assess the safety and immunogenicity of their COVID-19 DNA candidate vaccines, SCOV1 and SCOV2. The vaccines were exclusively administered using the PharmaJet Tropis® and Stratis® needle-free precision delivery systems.
By PharmaJet · Via Business Wire · March 21, 2023

PharmaJet®, a company that engineers precision delivery systems that overcome the challenges of vaccine delivery, today announced the December 2022, in-country kickoff of a study to evaluate the impact of intradermal (ID) vaccine administration using their Tropis® ID Needle-free Injection System (NFIS). PharmaJet and co-investigators from the National Primary Health Care Development Agency (NPHCDA), Jhpiego, PATH and the Sydani Group met in Lagos Nigeria and have finalized the study design, outlined the timelines, protocol, and tools for the study.
By PharmaJet · Via Business Wire · March 7, 2023
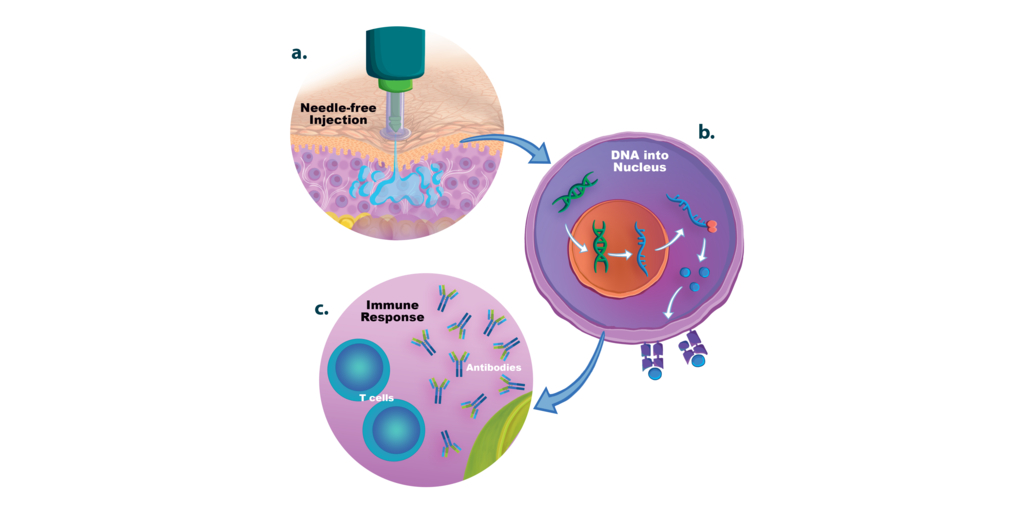
In a review article1 recently published in Vaccines Journal (MDPI), Dr. Carmen Ledesma-Feliciano, Scientific Affairs Manager at PharmaJet, referencing over 25 studies, discusses multiple injection delivery methods including PharmaJet’s needle-free precision delivery systems, which enhance the clinical performance of DNA-based vaccines.
By PharmaJet · Via Business Wire · February 23, 2023

PharmaJet®, a company that engineers precision delivery systems that overcome the challenges of vaccine and pharmaceutical companies, today announced that their partner Immunomic Therapeutics received FDA fast track designation (FTD) for the clinical study of their plasmid DNA vaccine ITI-3000 in patients with Merkel cell carcinoma (MCC), a rare but aggressive form of skin cancer. Enrollment is in progress for the phase 1 study that exclusively uses the PharmaJet Stratis Needle-free Injection System (NFIS).
By PharmaJet · Via Business Wire · December 13, 2022

PharmaJet®, a company that has developed and commercialized a needle-free platform to more effectively administer drugs and biologics, today announced they received an $800,000+ direct to Phase II grant from the National Institutes of Health (NIH), to evaluate the immunogenicity of intradermal administration of human papillomavirus virus (HPV) vaccine using their Tropis intradermal (ID) Needle-free Injection System (NFIS). The study will compare Tropis intradermal delivery against traditional needle and syringe (N/S) intramuscular administration.
By PharmaJet · Via Business Wire · November 29, 2022

PharmaJet®, a company that has developed and commercialized a needle-free platform to more effectively administer drugs and biologics, today announced that their PharmaJet Tropis® Needle-free Injection System (NFIS) will be used in a door-to-door immunization campaign aimed at reducing the outbreak of circulating mutant poliovirus Type 2 (cMPV2). The initial pilot, targeting thousands of children under 5 years of age in the Sokoto North local government area (LGA) of Nigeria, will begin on November 5, 2022 and run for 4 days.
By PharmaJet · Via Business Wire · November 8, 2022

PharmaJet®, a company that has developed and commercialized a needle-free platform to more effectively administer drugs and biologics, today announced they received a multi-year, $1.5 million grant from the United States Agency for International Development (USAID), to evaluate the impact of intradermal (ID) vaccine administration using their Tropis® Needle-free Injection System (NFIS). The project will measure vaccine coverage and cost using Tropis ID for fractional inactivated poliovirus vaccine (IPV) delivery compared to standard intramuscular delivery using needle and syringe (N/S).
By PharmaJet · Via Business Wire · October 24, 2022

PharmaJet®, a company who has developed and commercialized a needle-free platform to more effectively administer drugs and biologics, today announced that their latest research results will be presented at five upcoming conferences in October:
By PharmaJet · Via Business Wire · September 27, 2022

PharmaJet®, a company who has developed and commercialized a needle-free platform to more effectively administer drugs and biologics, today announced that they will present their latest research results at the Military Health System Research Symposium (MHSRS) on September 13, 2022. The presentation, entitled Advancement of a Next Generation Needle-Free Injection Device for Effective Delivery of the Venezuelan Equine Encephalitis Virus DNA Vaccine, will be presented by Erin Spiegel, Ph.D., Vice President of Clinical and Regulatory Affairs at PharmaJet. The MHSRS is the foremost scientific meeting for the Department of Defense providing a venue for presenting new scientific knowledge resulting from military-unique research and development.
By PharmaJet · Via Business Wire · September 12, 2022

PharmaJet®, a company who has developed and commercialized an intradermal needle-free platform to more effectively administer drugs and biologics, today announced that the World Health Organization (WHO) selected the PharmaJet Tropis® Needle-free Injection System (NFIS) for large polio vaccination campaigns in Pakistan as part of a broader effort of coordinated campaigns between Pakistan and Afghanistan. Over 2.7 million children have been targeted for this program so far using the PharmaJet Tropis® NFIS.
By PharmaJet · Via Business Wire · August 31, 2022

PharmaJet®, a company that has developed and commercialized a needle-free platform to more effectively administer drugs and biologics announced that its partner, University of Adelaide, has started a human trial of a DNA-based COVID-19 vaccine booster using the PharmaJet Tropis® Needle-free Injection System (NFIS).
By PharmaJet · Via Business Wire · August 16, 2022

PharmaJet®, a biotech company that, with their innovative needle-free technology, has developed a more effective way of administering drugs and biologics to accelerate research, commercialization and public health outcomes, announced that its partner, Scancell, will include needle-free delivery of their Phase 2 clinical study for treatment of patients with advanced melanoma.
By PharmaJet · Via Business Wire · August 2, 2022

PharmaJet®, a company who has developed and commercialized a needle-free platform to more effectively administer drugs and biologics, today announced that they will present their latest research results at the mRNA-Based Therapeutics Summit on July 27, 2022. The presentation, entitled Advances in mRNA vaccines and therapeutics delivery with PharmaJet Needle-free Injection Systems, will be presented by Carmen Ledesma-Feliciano, DVM, PhD, DACLAM, Clinical Device Specialist at PharmaJet. This event, which takes place in Boston, will convene mRNA experts to highlight emerging tools and technologies that support the development of scalable and commercially viable mRNA-based therapeutics.
By PharmaJet · Via Business Wire · July 19, 2022

PharmaJet®, a biotech company that has developed a more effective way of administering drugs and biologics with their innovative, needle-free injection technology, announced that its partner, Nykode Therapeutics announced positive interim results from its phase 2 trial of their novel candidate, VB10.16, in combination with checkpoint inhibitor atezolizumab for treating advanced cervical cancer. The DNA-based therapeutic cancer vaccine is delivered with the PharmaJet needle-free injection technology.
By PharmaJet · Via Business Wire · June 21, 2022

PharmaJet®, a biotech company that has developed a more effective way of administering drugs and biologics with their innovative, needle-free injection technology, today announced that the phase 3 clinical study sponsored by their partner, Zydus Lifesciences, has been published in The Lancet. The peer-reviewed article, entitled Efficacy, safety, and immunogenicity of the DNA SARS-CoV-2 vaccine (ZyCoV-D): the interim efficacy results of a phase 3, randomized, double-blind, placebo-controlled study in India, features ZyCoV-D, the world’s first plasmid-DNA vaccine approved for use in humans, delivered exclusively by the PharmaJet Tropis® Intradermal Needle-free Injection System.
By PharmaJet · Via Business Wire · June 7, 2022

PharmaJet®, a biotech company that has developed a more effective way of administering drugs and biologics with their innovative, needle-free injection technology, announced that its partner, the Institute for Molecular Medicine (IMM) was awarded a $12 million grant from the National Institute on Aging (NIA) division of the National Institutes of Health (NIH). The grant supports clinical trials of their beta-amyloid vaccines based on DNA and recombinant protein for the prevention of Alzheimer’s disease (AD). A phase 1 clinical study in the U.S. is expected to begin in second quarter 2022 and the vaccine will be exclusively delivered by the PharmaJet Tropis® Needle-free Injection System.
By PharmaJet · Via Business Wire · May 24, 2022

PharmaJet®, a biotech company that has developed a more effective way of administering drugs and biologics with their innovative, needle-free injection technology, today announced that Darin Zehrung has joined the executive leadership team as Chief Business Development Officer. Mr. Zehrung is a global health business executive with over 25 years of technical, business and research leadership experience, most notably with PATH, the global nonprofit improving public health. In this role he will lead the development of new business for the Company, advancing strategic partnerships with global health agencies such as GAVI, WHO, UNICEF, and CEPI, and establishing partnerships with private/public companies in the global health market to advance commercial opportunities.
By PharmaJet · Via Business Wire · May 10, 2022

PharmaJet®, a biotech company that has developed a more effective way of administering drugs and biologics with their innovative, needle-free injection technology, today announced that their latest research results were presented at the World Vaccine Congress on April 20. The presentation, entitled Benefits of Vaccine and Therapeutics Delivery with PharmaJet’s Needle-free Injection Systems, was presented by Carmen Ledesma-Feliciano, DVM, PhD, DACLAM, Clinical Device Specialist at PharmaJet, Inc.
By PharmaJet · Via Business Wire · April 27, 2022
PharmaJet®, a biotech company that has developed a more effective way of administering drugs and biologics with their innovative, needle-free injection technology, today announced the launch of their new website aimed at expanding partner support with on-line tools for more rapid and efficient pharmaceutical development. Focusing on PharmaJet’s mission, “Enabling greater access to life-improving pharmaceuticals”, the new website provides a better user experience, highlighting the improved performance that can be achieved with needle-free delivery and providing existing and potential partners easy access to valuable information and resources to help them make informed decisions.
By PharmaJet · Via Business Wire · April 13, 2022

PharmaJet®, a biotech company that has developed a more effective way of administering drugs and biologics with their innovative, needle-free injection technology, announced the release of a video highlighting the recent implementation of a polio vaccination campaign in Somalia.
By PharmaJet · Via Business Wire · March 29, 2022

PharmaJet®, a biotech company that has developed a more effective way of administering drugs and biologics with their innovative, needle-free injection technology, announced that its partner Immunomic Therapeutics will be starting a phase 1 clinical study of their plasmid DNA vaccine ITI-3000 in patients with Merkel cell carcinoma (MCC), a rare but aggressive form of skin cancer. The study will be conducted at the University of Washington School of Medicine and the Fred Hutchinson Cancer Research Center in Seattle, Washington and will exclusively use the Stratis® Needle-free injection System for delivery of the vaccine.
By PharmaJet · Via Business Wire · March 15, 2022

PharmaJet®, a biotech company that has developed a more effective way of administering drugs and biologics with their innovative, needle-free injection technology, announced that their partner, Technovalia, has reported interim safety results from their needle-free SARS-CoV2 DNA vaccine phase 1 trial. Interim safety data of COVIGEN, their DNA COVID-19 vaccine candidate, is well tolerated and no safety concerns were observed.
By PharmaJet · Via Business Wire · February 15, 2022

PharmaJet®, a biotech company that has developed a more effective way of administering drugs and biologics with their innovative, needle-free injection technology, announced that Chris Cappello, President and CEO of PharmaJet was featured in CEOCFO Magazine.
By PharmaJet · Via Business Wire · February 1, 2022

PharmaJet®, the maker of innovative, needle-free injection technology, today announced that its Needle-free Injection System (NFIS) will be used in a clinical trial to deliver a booster vaccine against COVID-19 virus variants. The vaccine developed by DIOSynVax, a spinoff company supported by the University of Cambridge in the UK, recently announced the start of the Phase 1 clinical trial. The vaccine will be delivered exclusively with the PharmaJet Tropis® Needle-free injection system which was selected based on its characteristic ability to increase the efficacy of nucleic acid-based vaccines and therapeutics. This is the 20th COVID-19 vaccine development program using PharmaJet Needle-free Injection Systems as their method of delivery.
By PharmaJet · Via Business Wire · January 18, 2022

PharmaJet®, the maker of innovative, needle-free injection technology, today announced that its partner Zydus Cadila, is partnering with Enzychem Lifesciences to manufacture their COVID-19 plasmid DNA vaccine (ZyCoV-D®) in Korea. The plan is to manufacture 80+ million doses of the ZyCoV-D vaccine, which was recently granted emergency use approval (EUA) by India’s national regulatory agency for those aged 12 years and above. The vaccine will be manufactured in Korea and exported to several lower-middle income countries in Latin America and Asian New Southern Policy member countries. It is exclusively delivered by the PharmaJet Tropis® Needle-free Injection System.
By PharmaJet · Via Business Wire · January 4, 2022

PharmaJet®, the maker of innovative, needle-free injection technology, today announced that one of its pharmaceutical partners, Nykode Therapeutics (formerly Vaccibody) has begun a phase 1/2 clinical trial to specifically address emerging SARS-CoV-2 variants. The DNA-based vaccines will be delivered intramuscularly in the clinical trial exclusively using the PharmaJet Stratis® Needle-free Injection System.
By PharmaJet · Via Business Wire · December 14, 2021

PharmaJet®, the maker of innovative, needle-free injection technology, has been named “Asia’s Most Innovative Vaccine Delivery System” at the Asia-Pacific Vaccine Excellence Awards 2021 (AVEA 2021). The award was accepted by Paul LaBarre, MS, MBA, Vice President, Global Business Development, PharmaJet, Inc.
By PharmaJet · Via Business Wire · November 30, 2021

PharmaJet®, the maker of innovative, needle-free injection technology, announced that their latest research results will be presented at the Vaccine World Asia Congress 2021 on November 17. The presentation, entitled Improving Vaccine Performance with Needle-free Delivery, is being presented by Paul LaBarre, MS, MBA, Vice President, Global Business Development. The presentation will include collaborative data about needle-free delivery of novel vaccines against polio, rabies, and COVID-19 that demonstrates how PharmaJet’s technology can be used to improve vaccine efficacy, increase campaign coverage, and elevate the patient and caregiver experience.
By PharmaJet · Via Business Wire · November 15, 2021

PharmaJet®, the maker of innovative, needle-free injection technology, today announced that its Tropis® intradermal needle-free injection system is being used in a campaign in Somalia delivering inactivated polio vaccine to more than 110,000 children. The purpose of the campaign, funded in part by the Bill and Melinda Gates Foundation, using PharmaJet’s needle-free delivery product, supplied by the World Health Organization (WHO), is to ensure children under 5 who missed their routine immunization, receive their full regimen of inactivated polio vaccine. A second dose will be given with the PharmaJet Topis device after one month.
By PharmaJet · Via Business Wire · October 21, 2021

PharmaJet®, the maker of innovative, needle-free injection technology, today announced that its partner, Scancell, has selected its devices to administer its two SARS-CoV-2 DNA vaccine candidates, SCOV1 and SCOV2. The vaccines will be exclusively administered using the PharmaJet Tropis® and PharmaJet Stratis® Needle-free Injection Systems.
By PharmaJet · Via Business Wire · September 14, 2021
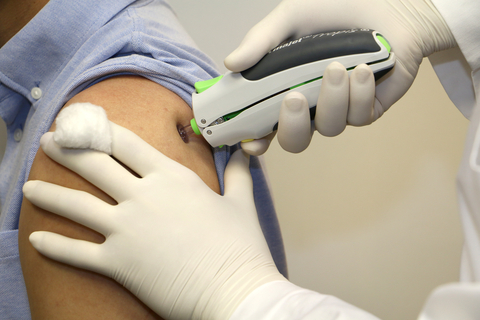
PharmaJet®, the maker of innovative, needle-free injection technology, today announced that its partner, Zydus Cadila, has received the Emergency Use Authorization (EUA) from the Drug Controller General of India (DCGI) for ZyCoV-D the world’s first Plasmid DNA Vaccine for COVID-19. The vaccine, ZyCoV-D, is exclusively administered using the PharmaJet Tropis® Needle-free Injection System.
By PharmaJet · Via Business Wire · August 23, 2021

PharmaJet®, the maker of innovative, needle-free injection technology, today announced that its partner Zydus Cadila has applied for Emergency Use Authorization (EUA) to the office of the Drug Controller General of India (DCGI) for its plasmid DNA Vaccine against COVID-19. The vaccine, ZyCoV-D, will be exclusively administered using the PharmaJet Tropis® Needle-free Injection System. Zydus Cadila is a global pharmaceutical company based in India, that develops, manufactures, and markets a broad range of healthcare therapies, including small molecule drugs, biologic therapeutics, and vaccines.
By PharmaJet · Via Business Wire · July 7, 2021

PharmaJet®, the maker of innovative, needle-free injection technology, today announced that patient enrollment has started for the phase 1 trial in Australia using its Needle-free Injection Systems to deliver a vaccine against SARS-CoV-2. COVIGEN, a DNA-based vaccine was developed by French-Thai pharmaceutical company BioNet-Asia in collaboration with and Melbourne-based biotech Technovalia. The PharmaJet System was chosen due to its record of increasing the effectiveness of DNA vaccines, ease-of-use, and speed of the delivery system compared to traditional needle and syringe.
By PharmaJet · Via Business Wire · June 30, 2021

PharmaJet®, the maker of innovative, needle-free injection technology, today announced that Paul LaBarre has joined the leadership team as Vice President, Global Business Development. Mr. LaBarre is a global business executive with over 20 years of global health experience across the innovation value chain including strategy, research, product development, and strategic business development, most notably with UNICEF and PATH. In this role he will be leading business development and commercialization strategies for the PharmaJet organization replacing Melissa Malhame who transitioned to a strategic advisory role for the company.
By PharmaJet · Via Business Wire · June 15, 2021

A systematic review and meta-analysis published in the April 30, 2021 issue of The Lancet strengthens evidence from previous publications that fractional dose delivery is a viable alternative to full dose inactivated polio vaccine (IPV). Previous studies have shown that two doses of intradermally-delivered IPV (60% less vaccine) were more immunogenic than one full dose delivered intramuscularly. Intradermal fractional dose delivery also lowers the total cost of vaccination substantially.
By PharmaJet · Via Business Wire · June 3, 2021
